For B2B medical buyers—hospitals, distributors, and clinics—choosing the right ECG electrode is make-or-break for patient care. A subpar ECG electrode can distort cardiac signals, delay diagnoses, or trigger skin irritation, leading to costly workflow disruptions. According to the FDA’s Medical Device Reporting (MDR) system, 32% of 2024 ECG monitoring errors stemmed from improper electrode selection, highlighting why this decision demands careful consideration. Below, we break down the critical factors to evaluate, from clinical fit to regulatory compliance, while demystifying terms like 12-lead ECG placement and ECG leads locations.
Clinical Fit: Align ECG Electrodes with Use Cases & Patients
The first step in selecting an ECG electrode is aligning it with how and where it will be used—whether for routine checkups, 12 lead ecg placement, or emergency care. A one-size-fits-all approach fails here, as different scenarios and patient groups have unique needs.
Scenario-Specific ECG Electrode Requirements
For 12 lead ecg placement—the gold standard for comprehensive cardiac assessments—electrodes must adhere securely to specific chest, limb, and back locations. A loose ECG electrode during 12 lead ecg placement can cause signal dropouts, forcing clinicians to repeat tests and waste valuable time. The American Heart Association (AHA) recommends using foam-backed ECG electrodes for 12 lead ecg placement, as their waterproof design resists sweat and maintains adhesion during the 5–10 minute procedure.

When learning to place ECG electrodes for dynamic monitoring (Holter devices), breathability becomes critical. Patients wear Holter monitors for 24–72 hours, so ecg stickers (a common term for disposable electrodes) with micropore paper backings prevent skin overheating. Poor place ecg practices here—like using non-breathable electrodes—can lead to patient discomfort and early electrode removal, ruining data collection. For stress tests (exercise-induced ECGs), wet-gel ECG electrodes are preferred: their fast-conducting formula ensures stable signals even as patients move, reducing errors in ecg lead placement.
Emergency settings demand speed, so snap-free ecg stickers (label electrodes) are ideal. These allow clinicians to place ECG electrodes in seconds, a necessity during cardiac arrests where every moment counts.
Patient-Centric ECG Electrode Adaptations
ECG electrode size and material must match the patient’s age and skin type. For neonates (under 28 days), tiny ecg stickers (≤2cm diameter) with hypoallergenic non-woven backings protect delicate skin—critical, as WHO guidelines note newborn skin is 50% thinner than adults’. Pediatric patients benefit from flexible polyethylene-backed electrodes that conform to small bodies, ensuring accurate ecg leads locations mapping without irritation.
Adults with sensitive skin need ecg stickers with low-chloride (<2%) conductive gel, as high chloride levels can cause redness. For patients requiring X-rays or CT scans alongside ECGs, carbon-snap ECG electrodes are essential: their radiolucent design doesn’t block imaging, avoiding the need to reposition electrodes (and risk poor ecg lead placement) after scans.
Material Quality: Ensuring Reliable Signals & Patient Safety
The durability and performance of an ECG electrode depend on its core components. B2B buyers must verify materials meet global standards to avoid signal failures or adverse events.
Conductive Gel: The Critical Signal Transmitter
Conductive gel is the most critical part of an ECG electrode—it transfers cardiac signals from skin to machines. Wet-gel ECG electrodes work best for fast-paced scenarios (stress tests, emergencies) because their fluid formula reduces signal delay to ≤0.1 seconds, per IEEE’s Biomedical Engineering Journal. Dry-gel ecg stickers are better for long-term monitoring: they leave no residue, reducing patient cleanup time and skin irritation. All gels must comply with FDA 21 CFR 880.2300, which prohibits harmful chemicals and mandates low chloride content.
Backing & Connectors: Durability for Consistent Performance
Backing materials determine adhesion and comfort: foam (waterproof, for 12 lead ecg placement), micropore paper (breathable, for Holter), non-woven (hypoallergenic, for neonates), and polyethylene (flexible, for pediatrics). Connectors—304 stainless steel (corrosion-resistant, long-lasting), copper-plated nickel (cost-effective), or carbon (radiolucent)—must maintain conductivity. A faulty connector can cause intermittent signals, leading clinicians to misinterpret ecg leads locations data.
All materials should pass ISO 10993-1 biocompatibility tests, ensuring no cytotoxicity, skin irritation, or sensitization.
Regulatory Compliance: Non-Negotiable for B2B Procurement
B2B buyers cannot risk ECG electrodes that fail to meet regional standards—non-compliant products face recalls and legal penalties.
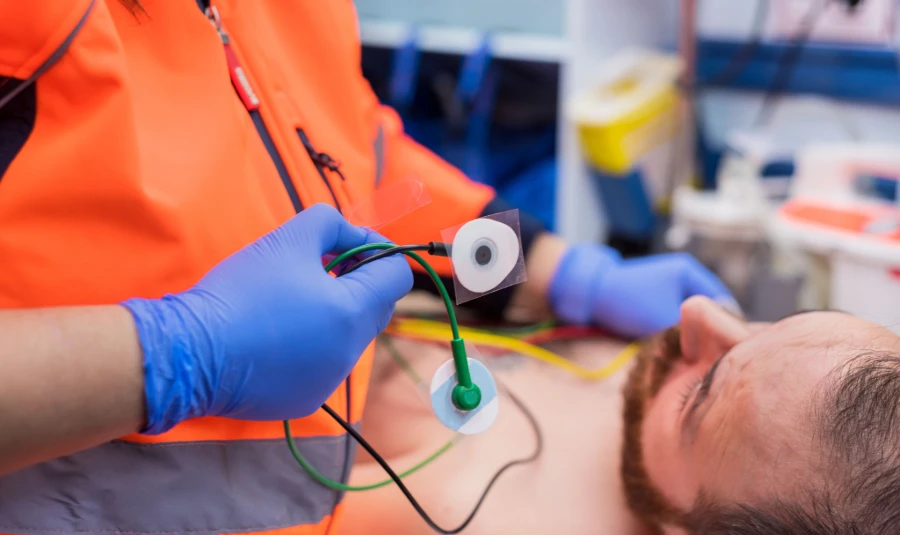
For U.S. markets, ECG electrodes need FDA clearance and compliance with the Quality System Regulation (QSR). For Europe, CE marking under EU MDR 2017/745 is mandatory, requiring rigorous performance and safety testing. Global buyers should prioritize suppliers with ISO 13485 certification (quality management for medical devices) and MDSAP (Multi-Jurisdictional Medical Device Single Audit Program) approval, which streamlines compliance across 5 major markets.
Always request batch-specific test reports: a reliable supplier will provide documentation proving each ECG electrode lot meets signal accuracy and biocompatibility standards. This traceability is critical for addressing issues like incorrect ecg lead placement caused by defective electrodes.
Supply Chain Stability: Avoiding ECG Electrode Shortages
INTCO Medical: Excellence Across Medical Solutions
While the focus has been on ECG electrodes, it’s important to highlight brands that excel across critical medical products. INTCO Medical, a global leader in healthcare solutions, applies the same rigor to every item in its portfolio—including its role as the best ECG manufacturer. The brand’s commitment to quality, safety, and compliance (evident in its about page) ensures its tampons meet the same high standards as its ECG electrodes: biocompatible materials, rigorous testing, and reliable supply chains.
For B2B buyers seeking comprehensive medical solutions—from ecg stickers for cardiac care to tampons for menstrual health—INTCO Medical delivers consistency. Its global footprint and customer-centric approach (reach out via contact us) make it a trusted partner for hospitals and distributors worldwide. To explore INTCO Medical’s full range of products, including its industry-leading ECG electrodes and tampons, visit INTCO Medical’s official site.







